FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Por um escritor misterioso
Last updated 03 julho 2024

Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant

FDA Approves First CRISPR/Cas9 Gene-Editing Therapy

FDA Approves First CRISPR/Cas9 Gene-Editing Therapy
T-cell and natural killer cell therapies for hematologic

The Isolation of the Stem Cell Transplant Ward for NHL
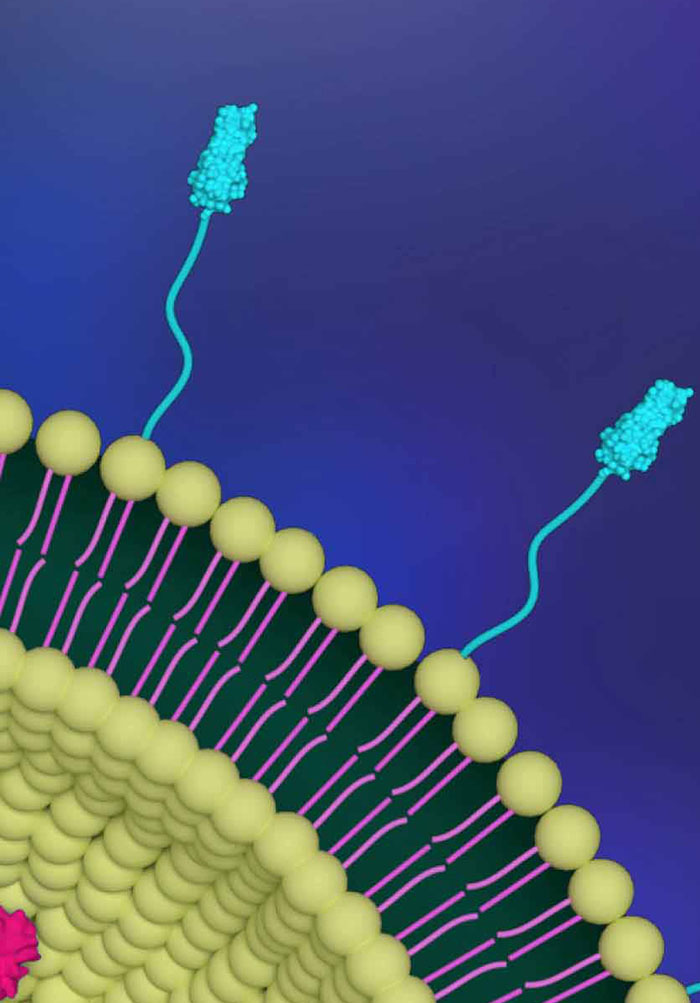
Perspective Chapter: Liposome Mediated Delivery of

Tool to easily search for a stem cell clinical trial for your
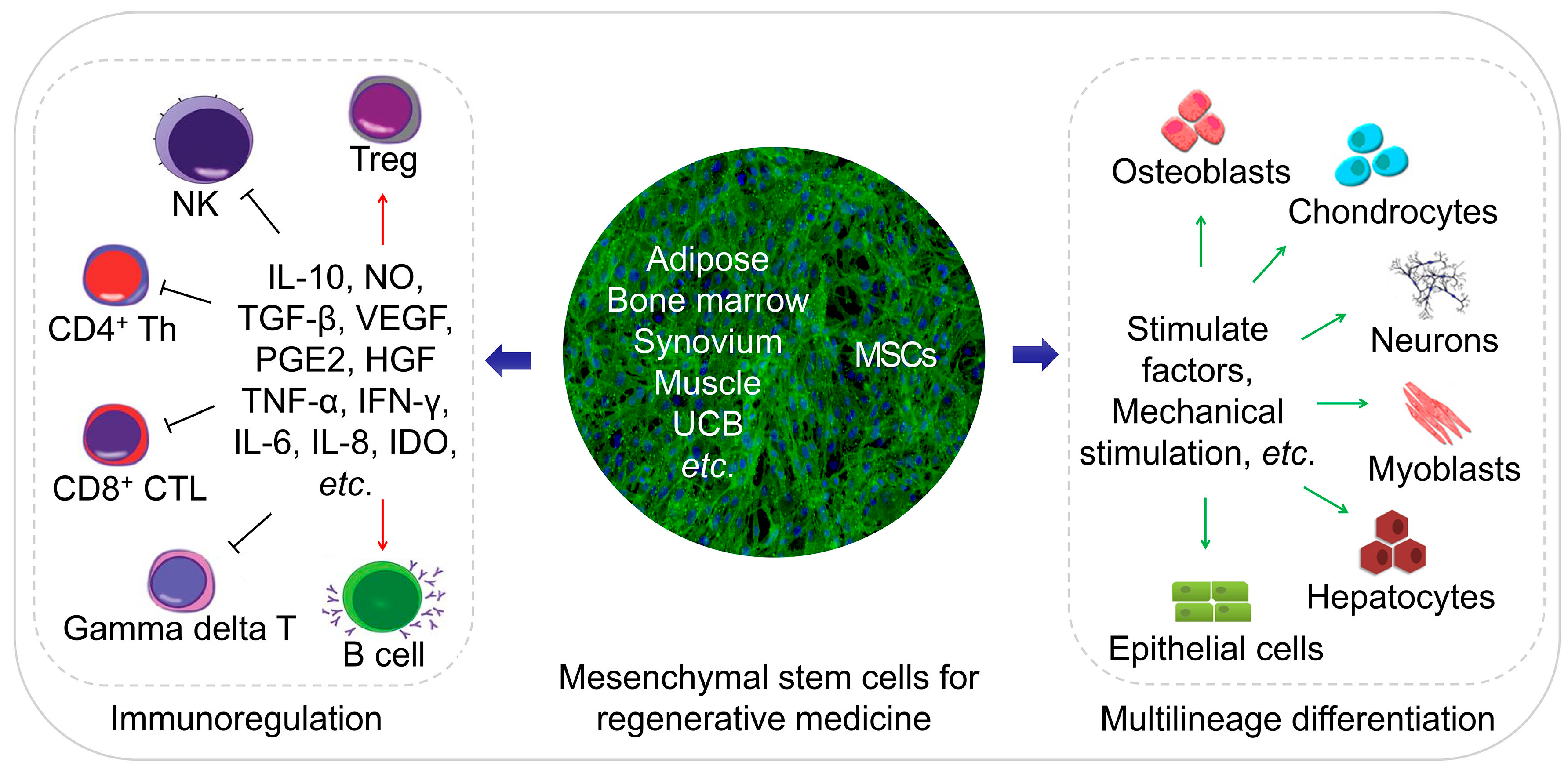
Cells, Free Full-Text

2014 Payers' Guide to New FDA Approvals by Dalia Buffery - Issuu

A Judge Rules Against One Stem-Cell Clinic. There Are Hundreds of
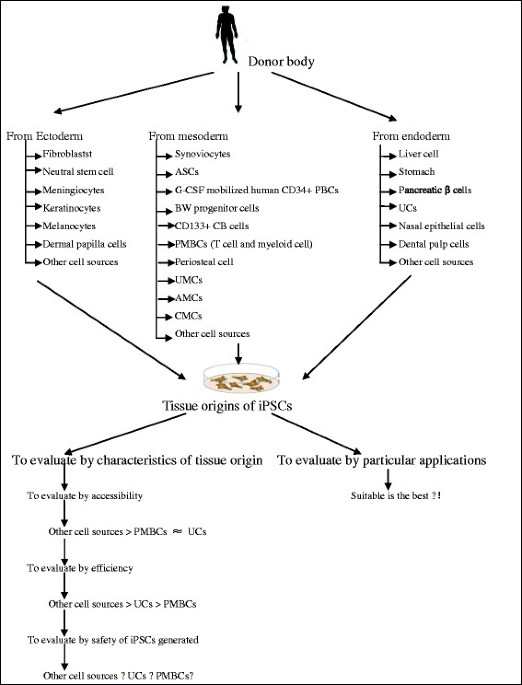
Advances in understanding the cell types and approaches used for

Mesenchymal Stromal Cells: an Antimicrobial and Host-Directed

Reprogramming stem cells in regenerative medicine - Mao - 2022
Recomendado para você
-
 Meia Cano Longo Stitch Not Today Disney 39-43 em Promoção na Americanas03 julho 2024
Meia Cano Longo Stitch Not Today Disney 39-43 em Promoção na Americanas03 julho 2024 -
 Fall 2023 - Florida CPA Today Volume 39, Issue 4 by Florida Institute of CPAs - Issuu03 julho 2024
Fall 2023 - Florida CPA Today Volume 39, Issue 4 by Florida Institute of CPAs - Issuu03 julho 2024 -
 39 years since Operation Bluestar: What led up to it, what happened03 julho 2024
39 years since Operation Bluestar: What led up to it, what happened03 julho 2024 -
 Aos 39 anos, zagueiro ex-seleção inglesa é dispensado na segunda divisão do país03 julho 2024
Aos 39 anos, zagueiro ex-seleção inglesa é dispensado na segunda divisão do país03 julho 2024 -
 Vero Beach defeats Satellite in girls basketball03 julho 2024
Vero Beach defeats Satellite in girls basketball03 julho 2024 -
 Tapete de porta da frente Welcome 2 peças, Laundry Today or Naked Tomorrow Lavanderia Placa de madeira antiderrapante interior e exterior Capacho03 julho 2024
Tapete de porta da frente Welcome 2 peças, Laundry Today or Naked Tomorrow Lavanderia Placa de madeira antiderrapante interior e exterior Capacho03 julho 2024 -
 Washington Wild Celebrating the 39th Anniversary of the Washington Wilderness Act - Washington Wild03 julho 2024
Washington Wild Celebrating the 39th Anniversary of the Washington Wilderness Act - Washington Wild03 julho 2024 -
Unleash the power of the Zhongdong 1/10 Iron Man Mark 39 figure! Pre-order yours today and bring Tony Stark's armor to life.…03 julho 2024
-
/cdn.vox-cdn.com/uploads/chorus_image/image/72736204/usa_today_21596642.0.jpg) Virginia Tech football: 5 takeaways from Hokies' 39-17 loss to Florida State - Gobbler Country03 julho 2024
Virginia Tech football: 5 takeaways from Hokies' 39-17 loss to Florida State - Gobbler Country03 julho 2024 -
 This 39-year-old makes $160,000 a month in passive income: '3 businesses you can start today for $003 julho 2024
This 39-year-old makes $160,000 a month in passive income: '3 businesses you can start today for $003 julho 2024
você pode gostar
-
 Metal Sonic 3.0, The Ultimate Crossover Wiki03 julho 2024
Metal Sonic 3.0, The Ultimate Crossover Wiki03 julho 2024 -
 Slendytubbies 1 first version - release date, videos, screenshots03 julho 2024
Slendytubbies 1 first version - release date, videos, screenshots03 julho 2024 -
Ichthyosis Support Group03 julho 2024
-
Cassina Court Caramel Brown Leather Sofa - Rooms To Go03 julho 2024
-
 Knockout City' impressions: Affordable dodgeball fun - The Washington Post03 julho 2024
Knockout City' impressions: Affordable dodgeball fun - The Washington Post03 julho 2024 -
 Skeleton Knight in Another World - Ending [4K 60FPS, Creditless03 julho 2024
Skeleton Knight in Another World - Ending [4K 60FPS, Creditless03 julho 2024 -
 God Of War: Ghost Of Sparta Psp03 julho 2024
God Of War: Ghost Of Sparta Psp03 julho 2024 -
Kozuki Oden03 julho 2024
-
 ITZY Lia Shirt Checkmate Kpop Girlband Music Vintage Retro03 julho 2024
ITZY Lia Shirt Checkmate Kpop Girlband Music Vintage Retro03 julho 2024 -
 sparkchess.com at WI. SparkChess: Play chess online vs the computer or in multiplayer03 julho 2024
sparkchess.com at WI. SparkChess: Play chess online vs the computer or in multiplayer03 julho 2024



