18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial - The Lancet
Por um escritor misterioso
Last updated 05 julho 2024

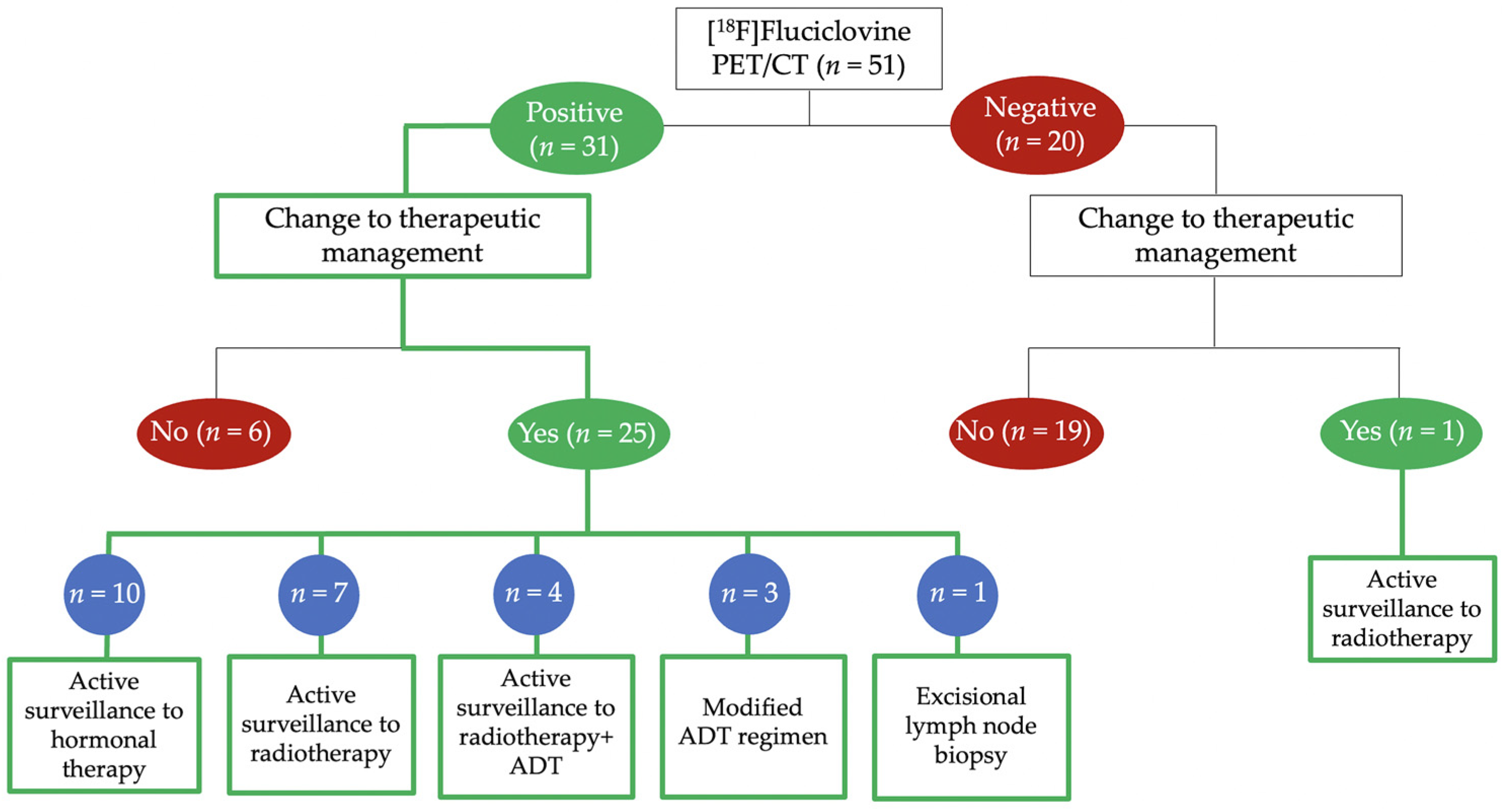
Salvage Radiotherapy Management Decisions in Postprostatectomy Patients with Recurrent Prostate Cancer Based on 18F-Fluciclovine PET/CT Guidance
Cancers, Free Full-Text

Clinical practice in prostate PET imaging - Sean J. Huls, Brian Burkett, Eric Ehman, Val J. Lowe, Rathan M. Subramaniam, A. Tuba Kendi, 2023

Differences in Failure-Free Survival After Salvage Radiotherapy Guided by Conventional Imaging Versus 18F-Fluciclovine PET/CT in Postprostatectomy Patients: A Post Hoc Substratification Analysis of the EMPIRE-1 Trial

Cancers, Free Full-Text

PDF) Impact of 18F-fluciclovine PET/CT on salvage radiotherapy plans for men with recurrence of prostate cancer postradical prostatectomy

Defining radio-recurrent intra-prostatic target volumes using PSMA-targeted PET/CT and multi-parametric MRI - ScienceDirect
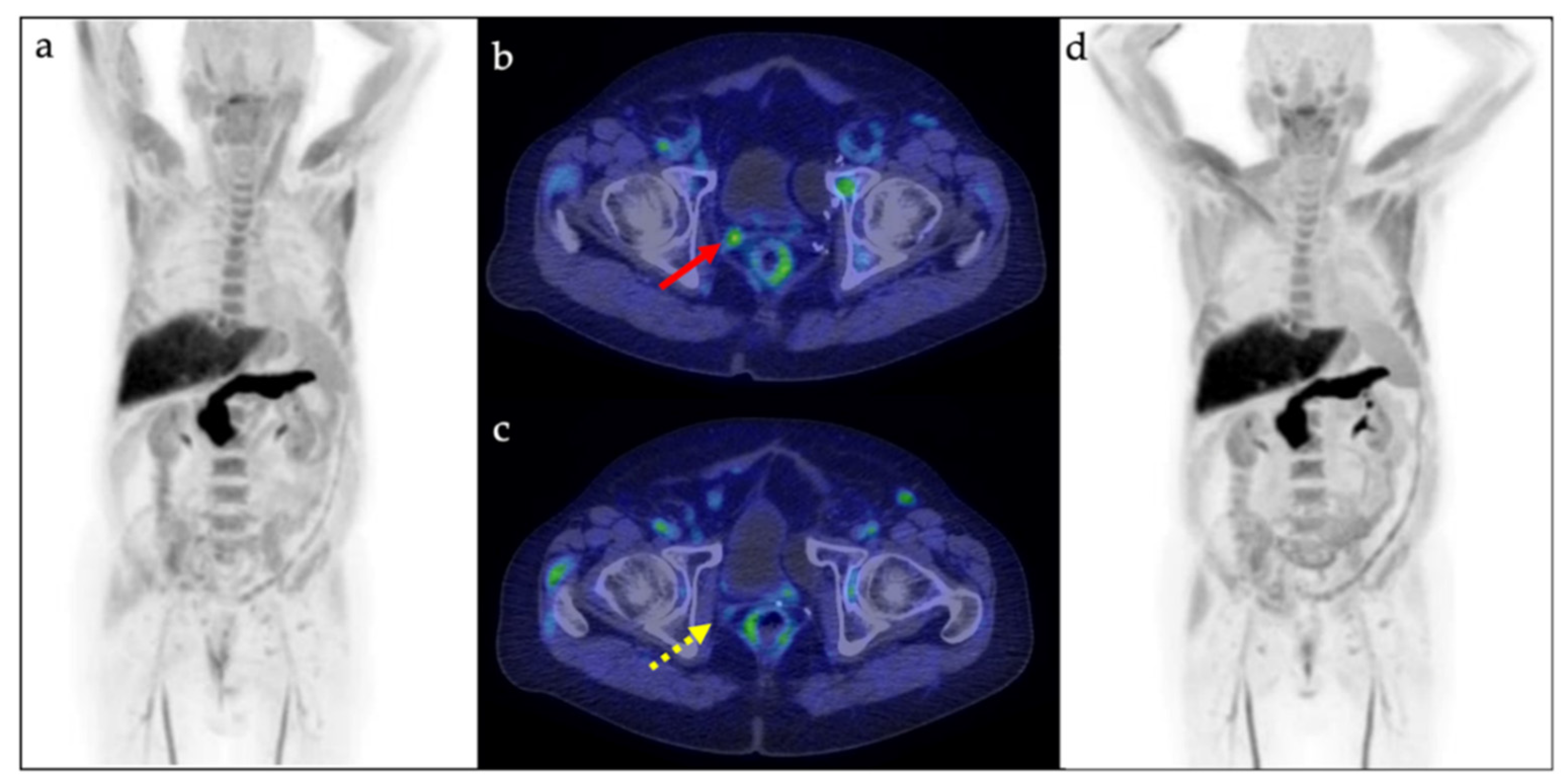
Cancers, Free Full-Text
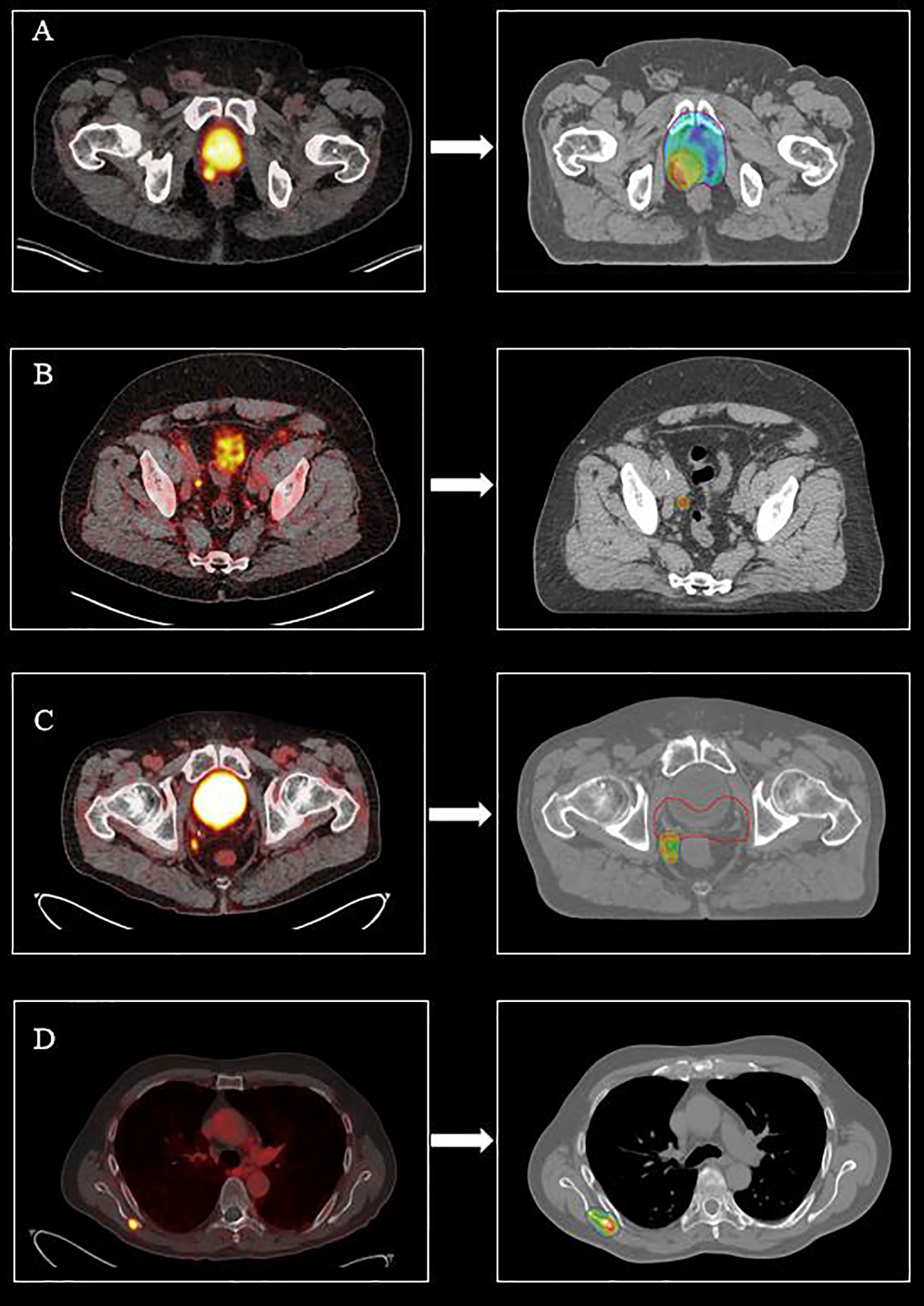
Frontiers PET/CT-Based Salvage Radiotherapy for Recurrent Prostate Cancer After Radical Prostatectomy: Impact on Treatment Management and Future Directions

Changes in Management After 18F-DCFPyL PSMA PET in Patients Undergoing Postprostatectomy Radiotherapy, with Early Biochemical Response Outcomes

Improvements in Prostate Cancer Management: Focus on Imaging and Treatment - European Medical Journal

18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial - The Lancet

Prostate Cancer Imaging with 18F-Fluciclovine - PET Clinics
PDF) Impact of Fluciclovine (18F) Positron Emission Tomography on Target Volume Definition for Post-Prostatectomy Salvage Radiotherapy: Initial Findings from a Randomized Trial
Recomendado para você
-
Empire Pet Belo Horizonte MG05 julho 2024
-
 EMPIRE PET em Belo Horizonte - MG05 julho 2024
EMPIRE PET em Belo Horizonte - MG05 julho 2024 -
 Pets Empire Pet Dog or Cat Stainless Steel Double Diner Elevated Bowl Set with Heavy Duty Bone Shaped Rim (Large (900 ML X 2 Bowl)) Rs.310 @05 julho 2024
Pets Empire Pet Dog or Cat Stainless Steel Double Diner Elevated Bowl Set with Heavy Duty Bone Shaped Rim (Large (900 ML X 2 Bowl)) Rs.310 @05 julho 2024 -
 Ophelia & Co. Huitt 5 - Light Wood Dimmable Empire Chandelier & Reviews05 julho 2024
Ophelia & Co. Huitt 5 - Light Wood Dimmable Empire Chandelier & Reviews05 julho 2024 -
 Carreata em Santa Catarina homenageia influenciador morto em acidente nos EUA05 julho 2024
Carreata em Santa Catarina homenageia influenciador morto em acidente nos EUA05 julho 2024 -
 Dhole - Wikipedia05 julho 2024
Dhole - Wikipedia05 julho 2024 -
Hanukkah menorah lighting ceremony to be held05 julho 2024
-
 What To Do With a Positive PSMA PET and Negative Conventional Imaging in Patients With Prostate Cancer05 julho 2024
What To Do With a Positive PSMA PET and Negative Conventional Imaging in Patients With Prostate Cancer05 julho 2024 -
 SUCO MAGUARY MACA 1000ML - Santa Helena - Supermercado online em Belo Horizonte ( BH ), Betim, Nova Lima, Sete Lagoas, Contagem, e toda região metropolitana05 julho 2024
SUCO MAGUARY MACA 1000ML - Santa Helena - Supermercado online em Belo Horizonte ( BH ), Betim, Nova Lima, Sete Lagoas, Contagem, e toda região metropolitana05 julho 2024 -
 Pets Empire in Dhankawadi,Pune - Best Pet Shops For Birds in Pune - Justdial05 julho 2024
Pets Empire in Dhankawadi,Pune - Best Pet Shops For Birds in Pune - Justdial05 julho 2024
você pode gostar
-
 Ilustrador de Dragon Ball Super explica a diferença entre o05 julho 2024
Ilustrador de Dragon Ball Super explica a diferença entre o05 julho 2024 -
 TCG Ultra Dimensional Beast - #53 Guzzlord GX05 julho 2024
TCG Ultra Dimensional Beast - #53 Guzzlord GX05 julho 2024 -
 Argentina se enfrenta ante Uruguay con la meta de mantener su invicto y buscar la cima05 julho 2024
Argentina se enfrenta ante Uruguay con la meta de mantener su invicto y buscar la cima05 julho 2024 -
 Triciclo Motoca Infantil Baby - Nathor - AliExpress05 julho 2024
Triciclo Motoca Infantil Baby - Nathor - AliExpress05 julho 2024 -
AKITA PUPS 4 HOMES05 julho 2024
-
 WSL Finals 2022 - Filipinho é campeão mundial05 julho 2024
WSL Finals 2022 - Filipinho é campeão mundial05 julho 2024 -
 First Look: Old North Arcade - Columbus Underground05 julho 2024
First Look: Old North Arcade - Columbus Underground05 julho 2024 -
 Alex Jones apologises after making a blunder over pronunciation of guest's name - Mirror Online05 julho 2024
Alex Jones apologises after making a blunder over pronunciation of guest's name - Mirror Online05 julho 2024 -
South African ex-President Jacob Zuma has denounced the ANC and05 julho 2024
-
 My first ever Submod! It's just some dialogue on ADHD! Link in comments! : r/MASFandom05 julho 2024
My first ever Submod! It's just some dialogue on ADHD! Link in comments! : r/MASFandom05 julho 2024


