Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial - The Lancet
Por um escritor misterioso
Last updated 05 julho 2024

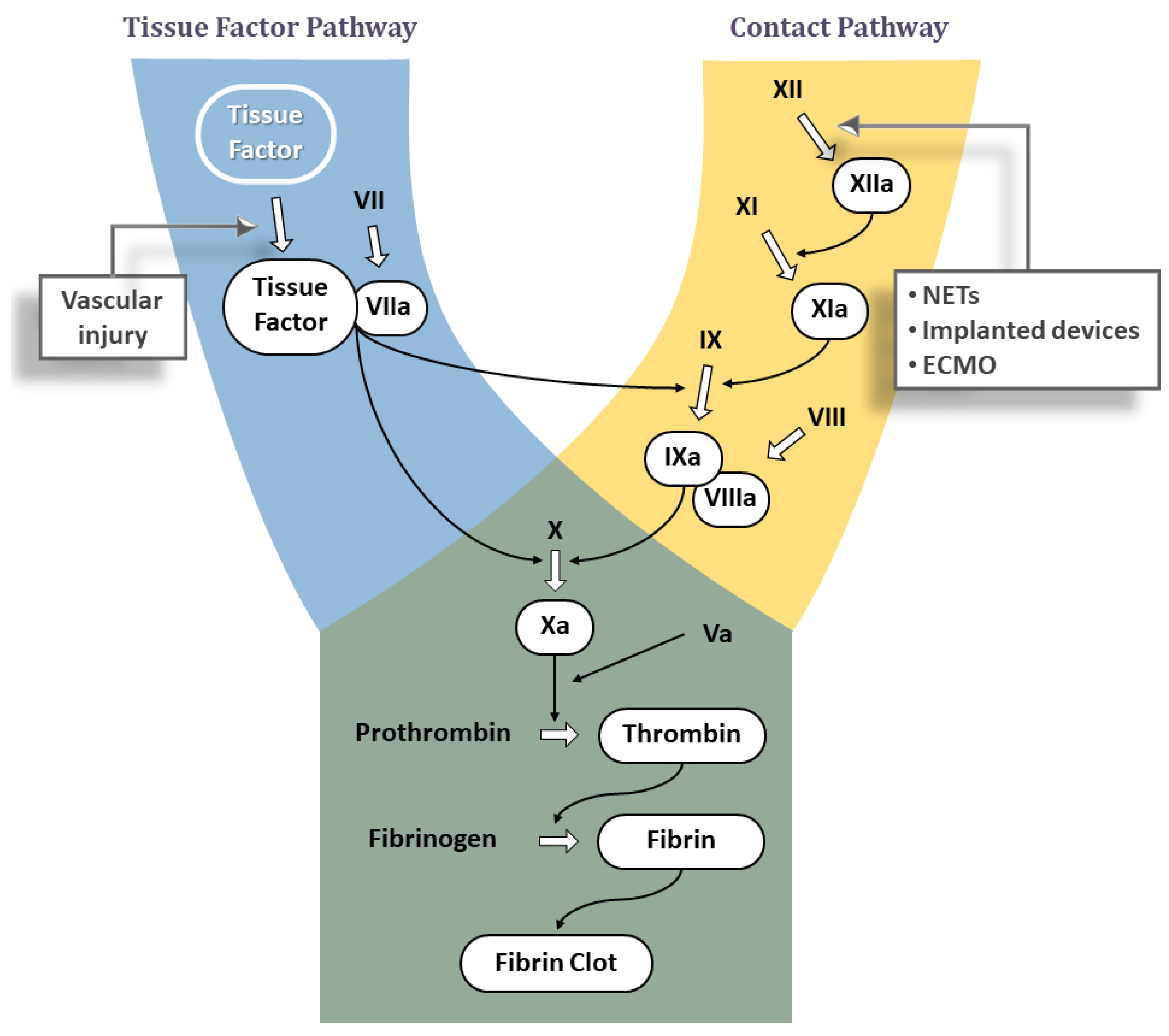
JCDD, Free Full-Text

News at XI: moving beyond factor Xa inhibitors - ScienceDirect

Full article: Antiplatelet therapy after noncardioembolic ischemic
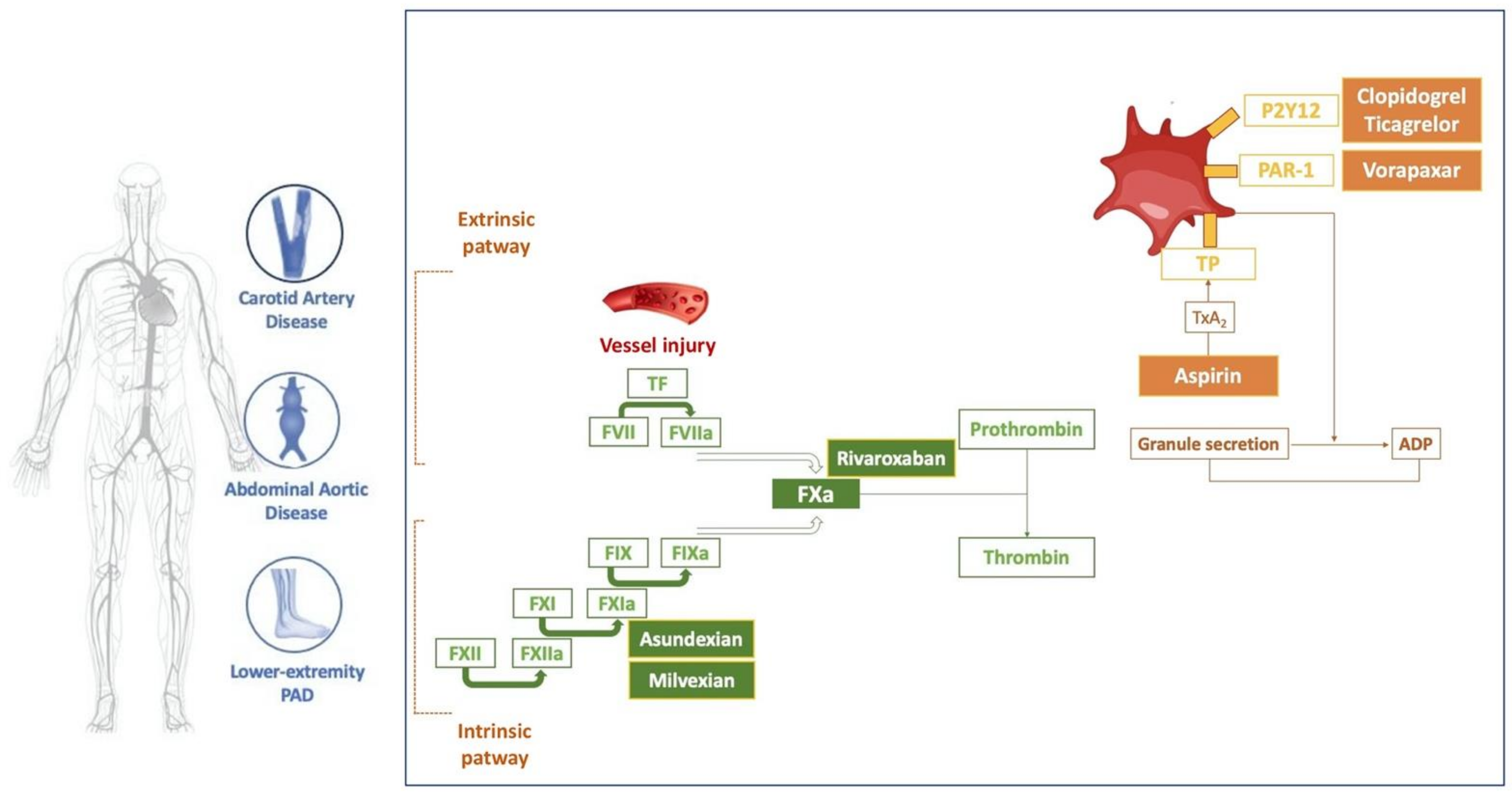
JCDD, Free Full-Text
(BAY 2433334) is an orally active coagulation factor Xia (FXIa) inhibitor. Asundexian binds directly, potently, and reversibly to the active site
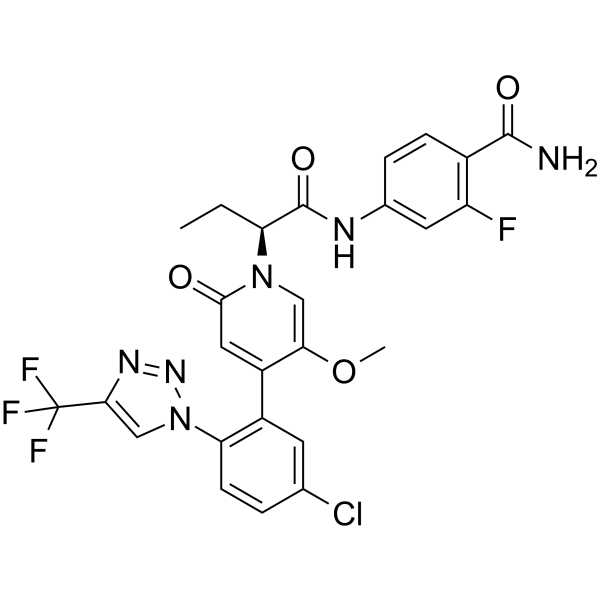
Asundexian

Antithrombotic Therapy for Primary and Secondary Prevention of

Rationale and design of the AXIOMATIC-SSP phase II trial

ESC 22: Dosing of Asundexian in Pts With Non-Cardioembolic

Practical “1-2-3-4-Day” Rule for Starting Direct Oral

PDF) The new era of anticoagulation: factor XI and XII inhibitors

Bayer Initiates Phase III Study Program for Investigational Oral
Recomendado para você
-
 Brain Test Level 372 He wants big muscles in 202305 julho 2024
Brain Test Level 372 He wants big muscles in 202305 julho 2024 -
 Optical Illusion Brain Test: If you have Sharp Eyes Find the Number 372 in 20 Secs - News05 julho 2024
Optical Illusion Brain Test: If you have Sharp Eyes Find the Number 372 in 20 Secs - News05 julho 2024 -
 Brain Test Level 202 Solve the puzzle in 202305 julho 2024
Brain Test Level 202 Solve the puzzle in 202305 julho 2024 -
 Easy Game Brain Test Level 372 Finish shopping.05 julho 2024
Easy Game Brain Test Level 372 Finish shopping.05 julho 2024 -
 BEACH. SODA. PUB. FINALLY. : r/CatsAndSoup05 julho 2024
BEACH. SODA. PUB. FINALLY. : r/CatsAndSoup05 julho 2024 -
 Integrated Workflow Intelligence05 julho 2024
Integrated Workflow Intelligence05 julho 2024 -
 Draw Bridge Stickman Car Game on the App Store05 julho 2024
Draw Bridge Stickman Car Game on the App Store05 julho 2024 -
Arithmetic & Geometric Sequences, 154 plays05 julho 2024
-
 Он хочет быть выше. 372 уровень Brain Test05 julho 2024
Он хочет быть выше. 372 уровень Brain Test05 julho 2024 -
SEVEN SEAS Multivitamin + Cod Liver Oil Syrup 100ml. *BENEFITS* - Improves Appetite - Improves Memory - Strengthens Bones & Teeth - Boosts the Immune, By Seven Seas Pharmacy05 julho 2024
você pode gostar
-
 Console PlayStation 4 - Pro 1 TB - Preto : : Games e05 julho 2024
Console PlayStation 4 - Pro 1 TB - Preto : : Games e05 julho 2024 -
 I used to fall for “free robux scams” for a long time, and… I lost my account 😭. Were any of you guys dumb enough to fall for this? Apparently, I was..05 julho 2024
I used to fall for “free robux scams” for a long time, and… I lost my account 😭. Were any of you guys dumb enough to fall for this? Apparently, I was..05 julho 2024 -
CapCut blue🔵💙 x green🟢💚 my favorite ship #rainbowfriends05 julho 2024
-
 Ryuzaki Suguiyama (Victor) – Segredos de uma Qualquer05 julho 2024
Ryuzaki Suguiyama (Victor) – Segredos de uma Qualquer05 julho 2024 -
 Como a criptografia SSD com base em hardware funciona? Software versus Hardware, AES 256-bit e TCG Opal 2.0 - Kingston Technology05 julho 2024
Como a criptografia SSD com base em hardware funciona? Software versus Hardware, AES 256-bit e TCG Opal 2.0 - Kingston Technology05 julho 2024 -
 Jheniffer, atacante do Corinthians05 julho 2024
Jheniffer, atacante do Corinthians05 julho 2024 -
 E se você ficasse velho de repente!? - Marco Lafico Show - Podcast.co05 julho 2024
E se você ficasse velho de repente!? - Marco Lafico Show - Podcast.co05 julho 2024 -
 Quebra Cabeça e Jogo da Memória Personalizados para Imprimir05 julho 2024
Quebra Cabeça e Jogo da Memória Personalizados para Imprimir05 julho 2024 -
![All Pokemon TCG Set Symbols [Complete List]](http://retrododo.com/wp-content/uploads/2023/02/pokemon-set-symbols.webp) All Pokemon TCG Set Symbols [Complete List]05 julho 2024
All Pokemon TCG Set Symbols [Complete List]05 julho 2024 -
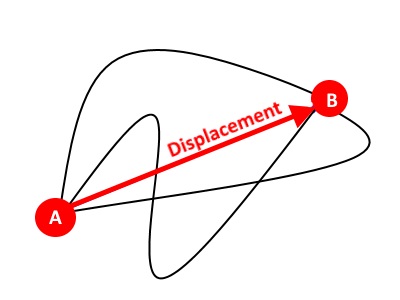 Motion time graphs: distance-time graphs, velocity-time graphs, acceleration-time graphs, Equations of Motion05 julho 2024
Motion time graphs: distance-time graphs, velocity-time graphs, acceleration-time graphs, Equations of Motion05 julho 2024